Concept of dyes
Dyes refer to colored organic compounds that can obtain colour and lustre from fiber materials, but not all colored organic compounds can be used as dyes. As a dye, there are four requirements.
1. Chroma
That is, it must be able to dye a certain concentration of color (a certain dyeing rate)
2. The ability to color.
That is to say, it has certain binding force with textile materials, namely affinity or directness.
3. Solubility
It can be dissolved directly in water or chemically in water.
4. Colour fastness
That is to say, the color dyed on textile materials should be durable and not easy to fade or discolor.
Some colored materials are insoluble in water, have no affinity for fibers and can not be included in the fibers, but they can be mechanically fixed on the fabric by the action of adhesives, which become pigments. Coatings can be prepared by grinding pigments and dispersants, moisture absorbents and water. Coatings can also be used for dyeing, but they are not widely used in printing.
Development and Classification of Dyes
In 1857, W. H. Perkin of Britain industrialized its aniline violet dye, which was the first synthetic dye.
Dyestuff production generally takes 1857 as the demarcation line: before 1857, it is the extraction and processing stage of natural dyes; after 1857, it is the production and processing stage of synthetic dyes.
According to the Dye Index, there are more than 7000 synthetic dyes (including organic pigments) in the world, and more than 2000 kinds of synthetic dyes are often produced. Synthetic dyes, though only 160 years old, have developed at an alarming rate.
Classification of dyes
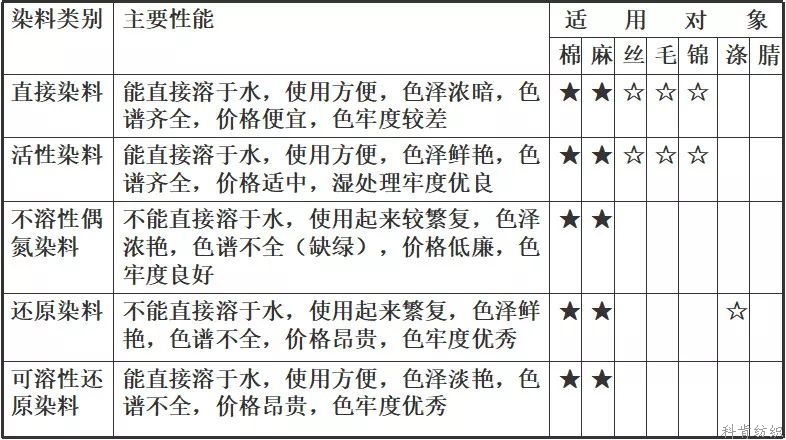
There are two kinds of classification methods for dyes. One is to classify dyes according to their properties and application methods, and the other is to classify dyes according to their chemical structure or characteristic groups, which is called chemical classification.
1. Classification by chemical structure
It can be divided into azo dyes, anthraquinone dyes, aromatic methane dyes, indigo dyes, sulfur dyes, phthalocyanine dyes, nitro and nitroso dyes. In addition, there are other types of dyes, such as methyl and polymethyl dyes, stilbene dyes and various heterocyclic dyes.
2 classification by application
Divided into: direct dyes, acid dyes, cationic dyes, reactive dyes, insoluble azo dyes, disperse dyes, VAT dyes, sulfur dyes, polycondensation dyes, fluorescent whitening agents, in addition, there are oxidizing dyes (such as aniline black), solvent dyes, polypropylene dyes and edible colors for food. Vegetarian and so on.
![]()
.bmp)
Nomenclature of dyes
There are many kinds of dyes. In order to meet the requirements of production and application, the proper color and application performance of reactive dyes must be given a special name for dyes.
According to the Draft Nomenclature of Dyestuff Products, which was piloted by the Ministry of Chemical Industry of China since January 1965, the nomenclature of dyestuffs in China, mdash; & mdash; three-stage nomenclature, is introduced as follows: the nomenclature of dyestuffs consists of three parts, namely, & ldquo; & ldquo; & rdquo; & ldquo; & rdquo; & ldquo; & ldquo; & rdquo;.
.bmp)
The Title part is mdash; & mdash; which denotes the application category of dyes, the commodity name or application category of dyes in the world denotes the title of dyes, and the application category or property of dyes is used as the title in China.
There are 31 kinds of dye crowns in China, such as acid, acid complexation, acid medium, neutral, direct, direct sunproof, direct blending, alkali, cation, activity, reduction, soluble reduction, sulfur, sulfur reduction, dispersion, chromophenol, chromophore, salt, condensation polycondensation, fast pigments, etc.

The color part - mdash; & mdash; indicates the color of dyes on fibers, basically the same at home and abroad.
Twenty-nine color names are used: tender yellow, yellow, golden yellow, dark yellow, orange, bright red, red, peach red, rose red, magenta, red purple, jujube red, purple, Turquoise blue, blue, Lake blue, brilliant blue, dark blue, green, brilliant green, dark green, yellow brown, brown, dark brown, olive green, grass green, grey, black.
The adjectives of color and lustre are used in three words: ldquo; tender & rdquo; & ldquo; Yan & rdquo; & ldquo; deep & rdquo; and so on.
The suffix - mdash; & mdash; that is to say, the color, shape, special properties and other dyeing properties are explained by certain symbols and figures.
Common Symbols and Their Significance Enumeration
(1) Quality indicating the colour or light of a dye
The customary names of dyes A are Hulan A and acid scarlet A.
B denotes blue or blue light-nbsp;
G denotes yellow or green light.
R denotes red light-nbsp;
F denotes colour and light purity-nbsp;
D means darker or darker.
Hellip; & hellip; & hellip; & hellip; & hellip;
(2) Representation of nature and use
AS insoluble azo dye chrysophanol series name, such as AS-D, etc.
B bis-triazine dyes, such as Megafix golden BES & nbsp;
C denotes resistance to chlorine bleaching, such as reductive blue BC, cotton; hydrochloride of insoluble azo dyesFor example, yellow-based GC
D is suitable for dyeing and high temperature resistant blended direct dyes, such as direct blending yellow D-RL, and high temperature disperse dyes, such as Dispersol orange D-G, can print VAT dyes, such as reduced brown RRD.
Hellip; & hellip; & hellip; & hellip; & hellip;
The suffix indicating dye strength-nbsp;
In the end note of dyestuff, dyestuff force is often expressed, such as 100%, 200%, etc. The so-called dye force refers to the relative concentration determined by the dye manufacturer with a certain mass fraction as the dye force standard (force as 100%). For example, 50% means that the strength of a dye is half that of a standard dye. The strength standards of different manufacturers are different and incomparable. Dyestuff factories often add fillers such as dyeing promoters, diffusives, cosolvents and other additives to the dyes. Attention should be paid to the use of these additives.
Illustrate with examples
Reactive Brilliant Red M-8B 150%, & ldquo; Reactive & rdquo; & ldquo; denotes reactive dyes as title; & ldquo; Brilliant Red & rdquo; denotes bright red after dyeing textile materials; & ldquo; M-8B 150%& rdquo; is the end of the word, & ldquo; M” refers to M-type reactive dyes, & ldquo; B” refers to the color and light of dyes. Blue, & ldquo; 8B” refers to the ratio of & ldquo; B” a lot of blue, indicating that this is a red dye with heavy blue light, & ldquo; 150% & rdquo; indicating the strength or strength of the dye.
[Life Analysis]
Azo is one of the compulsory items for international environmental protection requirements, but not all azo dyes are prohibited. Only 24 aromatic amine azo dyes designated by law can be released after reduction, about 130 of which are prohibited. After long-term contact with human skin, these Prohibited Azo Dyed clothing or other consumer goods will mix with the components released during metabolism and produce reductive reactions to form carcinogenic aromatic amine compounds, which will be absorbed by human body and make the structure and function of human cell DNA through a series of activation. Change is the inducement of human pathological changes.
Generally, there are three kinds of inspection methods: thin layer chromatography (TLC), gas chromatography-mass spectrometry (GC-MSD) and high performance liquid chromatography (HPLC). The standard stipulates that 23 kinds of azo dye intermediates should not be contained in the tested products. If one of them is detected, it will be an unqualified product with a limit of 30 ppm.
1. test method
The orange nylon fabric sample was used as the test object to carry out determinative and quantitative analysis.
![]()
2. main instruments
CP224S electronic balance; HD500 constant temperature water bath oscillator; American organomation nitrogen blower; Swiss BUCHI rotary evaporator; TGL-16B centrifuge; Agilent 7890A-5975C gas chromatography-mass spectrometer.
3. Major reagents and consumables
Tert-butyl methyl ether (TEDIA-HPLC); acetonitrile (TEDIA-HPLC); sodium dithionite (Chinese medicine-nbsp; AR); azo mixing standard (Beijing Zhenxiang-nbsp; 100ppm); diatomite column (Agela); pasteurized straw; 1.5mL centrifugal tube.
4. test principle
Textiles are reduced and decomposed in citrate buffer solution with sodium dithionite solution. Aromatic amines in the solution are extracted by tert-butyl methyl ether through diatomite column. After concentration, they are volumetrized with acetonitrile. Finally, samples are analyzed by GC-MS.
As aniline was detected, the new sample was reduced with sodium dithionite in alkaline medium, and 4-aminoazobenzene was extracted with tert-butyl methyl ether. The possible decomposition was qualitatively analyzed by GC-MS.
![叶子分割线]()
5. test the specific process.
5.1 Sample, replace the surface sample, cut into 5 mm & times; 5 mm & nbsp; small pieces, mix. 1.00G sample was weighed from the mixed sample, accurate to 0.01g, and put into the customized reactor.
![]()
5.2 Reduction, adding 17 mL of citrate buffer solution preheated to (70-plusmn; 2) (?) C, sealed, shaken and placed in (70-plusmn; 2) (?) C constant temperature water bath oscillator for 30 minutes, then opening the reactor, adding fresh 200 mg/L concentration of sodium dithionite solution 3 mL, and immediately closed shaking and then in (70-plusmn; 2) (?) C constant temperature water bath oscillator for 30 minutes, then opening the reactor, adding fresh 200 mg/L concentration of sodium dithionite solution 3 mL (70-plusmn; 2) The constant temperature water-bath oscillator is kept for 30 minutes and cooled to room temperature within 2 minutes after removal.

5.3 After cooling, the reaction solution was quickly poured into the diatomite column with glass rod, and then absorbed for 15 minutes, followed by 4-times. The 20 mL tert-butyl methyl ether was eluted four times, and the tert-butyl methyl ether extract was collected in a round-bottom flask.

5.4 Concentration. A round-bottomed flask was placed on a rotary evaporator and concentrated to about 1 mL at 40-50 C and 400-500 mbar pressure. It was then blown to near-dry by a nitrogen blower and volumetrized with 1 mL acetonitrile. After ultrasonic dissolution, the pasteurized pipette was moved to 1.5mL centrifugal tube for 3 min at 9000 rpm/separation center.
Rotational evaporation

Nitrogen blowing

centrifugal

5.5 Sampling was analyzed by Agilent 7890A-5975C gas chromatography-mass spectrometry (GC-MS). Sampling volume was 1μ L.
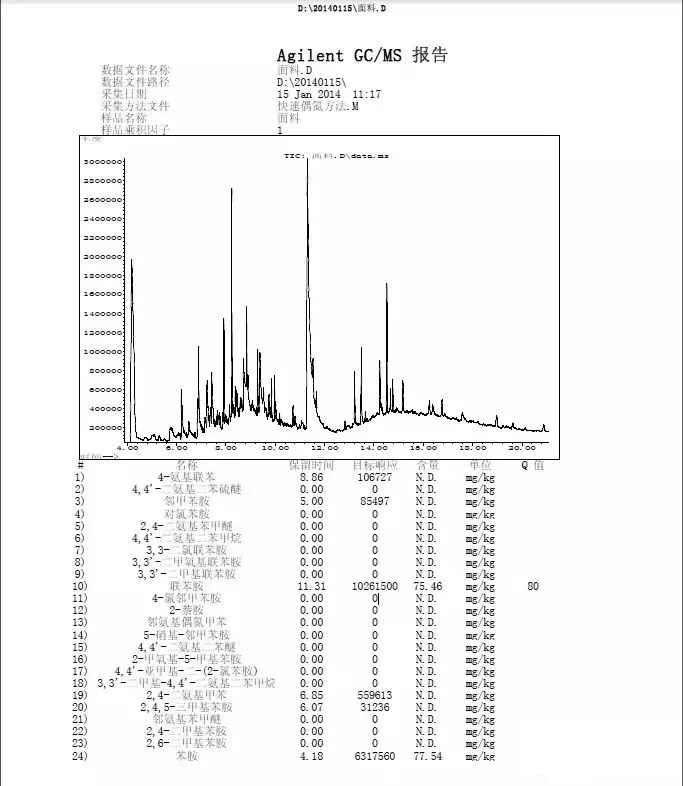
5.6 Preliminary results. In the analysis of results, aniline and benzidine were detected, and 4-aminoazobenzene should be detected according to GB/T 23344-2009.

5.74-AAB detection process: 1.00G sample was weighed from the mixed sample, accurate to 0.01g, put into the customized reactor, 9.0mL sodium hydroxide solution was added to the reactor, 1.0mL sodium dithionite solution was added to the closed shaking, and then placed in (40-plusmn; 2) - C constant temperature water bath oscillator for 30 minutes after closed shaking. Cool to room temperature within 1 minute after removal. 10 mL tert-butyl methyl ether and 7 g sodium chloride were added to the reactor in turn. After shaking forcefully, the reactor was shaken in a water-bath oscillator (room temperature) for 45 minutes. After static stratification, the upper organic phase is taken over 0.45-m.U; m membrane backward GC-MS analysis.
5.8 Final results: The unknown sample contained 75 mg/kg of benzidine and no 4-AAB.
6. points for attention:
6.1 Before making the sample (or before the unit is ready to carry out the azo project), there is a need for methodological development records. In other words, it is necessary to use the standard sample for recovery and other items to ensure the feasibility of the method.
6.2 Different diatomite columns have a great influence on the recovery rate. It is better to replace the diatomite columns and use different batches of diatomite columns before doing the recovery rate.
6.3 The tightness of the reactor also has a great influence on the recovery rate, which ensures that the reactor is tightly sealed.
6.4 In the process of concentration, the rotary evaporation must remain about 1 mL and must not be evaporated; the nitrogen blower needs to be blown to near-dry with slow nitrogen gas, not completely dry, otherwise the recovery rate will have a greater impact.
6.5 Ensuring the effectiveness of sodium dithionite has a great influence on the results. One way to judge the effectiveness is to reduce the discoloration of non-disperse dye samples. Another method is to configure fresh, colorless and transparent solution, which turns yellow as soon as it touches the air.

6.6 The time from cooling to column crossing is shorter, and the longer time will also affect the recovery rate. Especially when making 4-aminoazobenzene.
6.7 buffer solution needs to meet the requirements, pH should be around 6.0, and its pH also has an impact on the recovery rate.
When 4-aminoazobenzene is made in 6.8, the concentration and volume of sodium dithionite solution need to be accurate, which has a great influence on the recovery rate.
Note: External standard method is used in this test. The results usually need to be confirmed by liquid phase and quantified by liquid phase or internal standard method.
Question:
(1) How to prepare citrate buffer solution with pH=6.0?
(2) In operation 5.2, which tool is used to add 3mL sodium sulfate solution?
(3) Is it necessary to extrude the sample with a glass rod in step 5.3 of the operation so that the reduced mixed solution can flow out as far as possible from the textile residue?
(4) How to Volume 1 mL acetonitrile in step 5.4 of operation? What about centrifugation after volume setting?
(1) According to the standard configuration: 12.526g citric acid and 6.320g sodium hydroxide are dissolved in 1000 mL water (but I use the analytic pure citric acid and sodium hydroxide configuration, generally the pH is about 5.5, so sodium hydroxide generally needs to add more points, is purchasing high-grade pure, next time according to the standard configuration to see if it is about 6.0)
?
(2) Both pipette gun and pipette can be used < br/>.
(3) Yes, according to the national standard. (bs en 14362-1:2012 is poured over the diatomite column with the sample)
(4) Transfer the 1mL acetonitrile into the round bottom flask with the help of a little ultrasound. Because there is sometimes precipitation after dissolution, it can be centrifuged (or through membranes).
.bmp)
.bmp)